Time Marks Parameters
The PEA Plus and M-PEA Plus software packages extract chlorophyll fluorescence values from the recorded data from Handy PEA+, Pocket PEA and M-PEA chlorophyll fluorimeters at 5 pre-defined Time Marks. The times are:
 = 50 microseconds
= 50 microseconds = 100 microseconds
= 100 microseconds = (
= ( step) 300 microseconds
step) 300 microseconds = (
= ( step) 2 milliseconds
step) 2 milliseconds = (
= ( step) 30 milliseconds
step) 30 milliseconds
Chlorophyll fluorescence values at these Time Marks are used to derive a series of further biophysical parameters, all referring to time base 0 (onset of fluorescence induction), that quantify the photosystem II behaviour for (A) The specific energy fluxes (per reaction center) for:
- Absorption (
 )
)
- Trapping (
 )
)
- Dissipation (
 )
)
- Electron transport (
 )
)
and (B) the flux ratios or yields:
- Maximum yield of primary photochemistry (
 )
)
- Efficiency (
 ) with which a trapped exciton can move an electron into the electron transport chain further than
) with which a trapped exciton can move an electron into the electron transport chain further than 
- Quantum yield of electron transport (
 )
)
The concentration of active PSII reaction centers per excited cross section ( ) is also calculated.
) is also calculated.
Performance Index Parameters (OJIP Analysis)
The Performance Index ( ) is essentially an indicator of sample vitality. It is an overall expression indicating a kind of internal force of the sample to resist constraints from outside. It is a Force in the same way that redox potential in a mixture of redox couples is a force. Exactly the
) is essentially an indicator of sample vitality. It is an overall expression indicating a kind of internal force of the sample to resist constraints from outside. It is a Force in the same way that redox potential in a mixture of redox couples is a force. Exactly the  is a force if used on a log scale. Therefore we say:
is a force if used on a log scale. Therefore we say:

 is derived according to the Nernst equation. It is the equation which describes the forces of redox reactions and general movements of Gibbs free Energy in biochemical systems. Such a force (or potential = force) is defined as:-
is derived according to the Nernst equation. It is the equation which describes the forces of redox reactions and general movements of Gibbs free Energy in biochemical systems. Such a force (or potential = force) is defined as:-

where  is the fraction of a partner in the reaction
is the fraction of a partner in the reaction  to
to  . Therefore:
. Therefore:

and if you now convert to:

or for redox reactions

Now the total potential in a mixture is the sum of the individual potentials or:
 ….etc
….etc
In our case  (on an absorption basis or on a chlorophyll basis) has three components:
(on an absorption basis or on a chlorophyll basis) has three components:
The first component shows the force due to the concentration of active reaction centers

therefore:

 is a parameter of the
is a parameter of the  test and it is related to the force generated by the RC concentration per antenna chlorophyll.
test and it is related to the force generated by the RC concentration per antenna chlorophyll.
The second component is the force of the light reactions, which is related to the quantum yield of primary photochemistry:

The driving force of the light reactions is therefore:

The third component is the force related to the dark reactions (after  ). These are normal redox reactions in the dark.Expressed by the
). These are normal redox reactions in the dark.Expressed by the  test as:
test as:

Where  = relative variable fluorescence at 2 ms or at the step
= relative variable fluorescence at 2 ms or at the step  therefore:
therefore:

Therefore the force of the dark reactions is:

Now all three components together make:
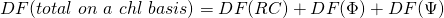
or without log

or in fluorescence terms:
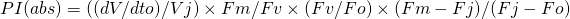
A more detailed derivation and explanation is beyond the scope and intention of this web page. Further detailed information may be obtained from the following publications which may be downloaded as PDF documents from the following links.
R.J. Strasser, A. Srivastava and M. Tsimilli-Michael
The fluorescence transient as a tool to characterize and screen photosynthetic samples.
Strasser, R.J., M. Tsimilli-Michael and Srivastava, A.
Analysis of the Fluorescence Transient.








 The sensor unit consists of an array of 3 ultra-bright red LEDs which are optically filtered to a peak wavelength of 650 nm (which is readily absorbed by chlorophyll) at a maximum intensity of up to 3,500 µmol m-2 s-1 at the sample surface. The LEDs are focused via lenses onto the leaf surface to provide uniform illumination over the area of leaf exposed by the leafclip (4mm dia). An optical feedback circuit monitors and corrects changes in the output intensity of the LEDs which can be caused by internal heat build-up within the LEDs themselves. The circuit also compensates for intensity changes caused by variation in ambient temperature.
The sensor unit consists of an array of 3 ultra-bright red LEDs which are optically filtered to a peak wavelength of 650 nm (which is readily absorbed by chlorophyll) at a maximum intensity of up to 3,500 µmol m-2 s-1 at the sample surface. The LEDs are focused via lenses onto the leaf surface to provide uniform illumination over the area of leaf exposed by the leafclip (4mm dia). An optical feedback circuit monitors and corrects changes in the output intensity of the LEDs which can be caused by internal heat build-up within the LEDs themselves. The circuit also compensates for intensity changes caused by variation in ambient temperature.